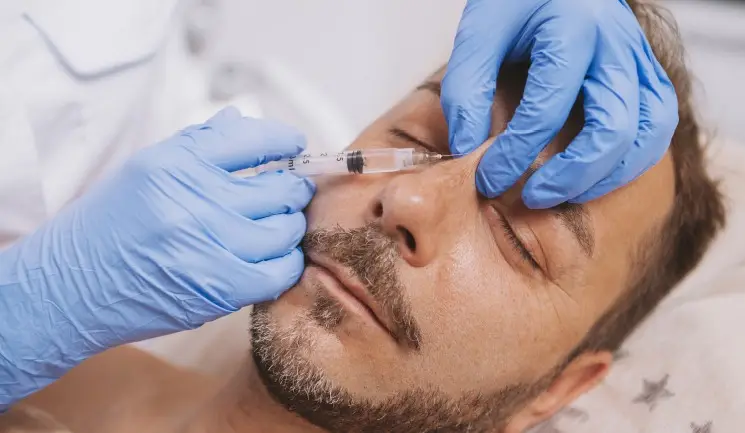
Mr Dominic Yue presents a case study demonstrating a non-surgical rhinoplasty complication and how it was effectively managed
To access this post, you must purchase Aesthetics Journal Membership – Annual Elite Membership, Aesthetics Journal Membership – Annual Enhanced Membership or Aesthetics Journal Membership – Basic Membership.
log in
log in