Neauvia receives new MDR certification
Global medical aesthetics business Neauvia has received a Medical Device Regulation (MDR) certification in accordance with new EU regulations.

Global medical aesthetics business Neauvia has received a Medical Device Regulation (MDR) certification in accordance with new EU regulations.

Skincare company LABthetics will unveil its LABpen microneedling device at CCR in October.
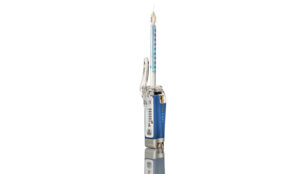
Swiss medical technology company Juvaplus will be launching a new robotic syringe for the use of botulinum toxin injections to the UK.

Aesthetic and medical supplier Ivanmed is launching the microneedling device Dermapen 4 at an event in London this month.
The Medicines and Healthcare products Regulatory Agency (MHRA) has revealed that models of Top-Q dermal filler are being sold with a falsely applied CE Mark.

The Advertising Standards Authority (ASA) has investigated a website and paid-for Google ad for Red Light Therapy.

To accompany the launch of new dermal filler Kysense, aesthetic company Circa Skin will host a webinar discussing its technology and techniques.

Microneedling device SkinPen Precision has been awarded a Class IIa CE Certification mark by the British Standards Institution (BSI).
Pharmaceutical company Croma Pharma has submitted its botulinum toxin drug file to the German Federal Institute for Drugs and Medical Devices (BfArM), for approval for the treatment of glabellar lines in the EU.
Aesthetic product manufacturer BioScience GmbH has received the European Commission approved CE mark extension for all its hyaluronic acid based dermal filler ranges HYAcorp, Genefill and HYAprof.