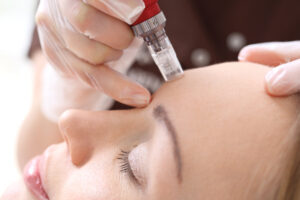
FDA warning on RF microneedling complications
The US Food and Drug Administration (FDA) has issued warnings to patients and healthcare providers after serious complications were reported from radiofrequency (RF) microneedling devices. According to the FDA, reported