Caromed Italia to launch skin booster range at ACE
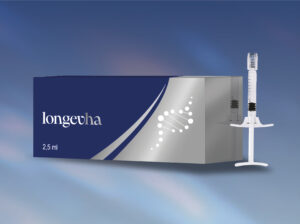
Aesthetic manufacturer Caromed Italia will debut three skin boosting products at ACE 2025 The company shares that it will be launching Evolutha – a medical device class 3 with fragmented

Aesthetic manufacturer Caromed Italia will debut three skin boosting products at ACE 2025 The company shares that it will be launching Evolutha – a medical device class 3 with fragmented

Communications consultant Julia Kendrick examines the regulations and guidelines for aesthetic marketing and advertising to the public

A study has found that a direct-to-consumer machine model for detecting skin cancers incorrectly classified rare and aggressive cancers as low-risk.

The Advertising Standards Authority (ASA) has investigated a website and paid-for Google ad for Red Light Therapy.

Microneedling device SkinPen Precision has been awarded a Class IIa CE Certification mark by the British Standards Institution (BSI).

Aesthetic device manufacturer 3D-Lipo Ltd has achieved the EN ISO 13485:2016 certification in line with the Medical Device Regulations that will be implemented on May 26.

Aesthetic manufacturers can now receive consulting and advice for hyaluronic acid and chitosan products with the launch of Falgagen.
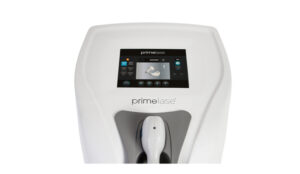
Aesthetic manufacturer Cocoon Medical has introduced hair removal device Primelase in the UK market.
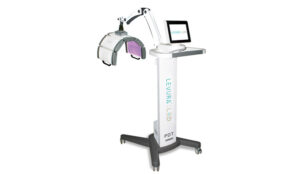
Medical equipment manufacturer Enoura Aesthetics has launched the Levura LED, the company’s first LED device.

Italian aesthetic manufacturer and distributor The Baldan Group introduced its two new releases, the Iridium Eyes Pressotherapy and InfraBaldan 3.0 as part of a two-day launch event held on January 20-21.