
FDA clears Tixel for skin resurfacing
Skin device Tixel, manufactured by Novoxel, has received clearance from the Food and Drug Administration (FDA) for dermatological procedures requiring ablation and resurfacing of the skin.

Skin device Tixel, manufactured by Novoxel, has received clearance from the Food and Drug Administration (FDA) for dermatological procedures requiring ablation and resurfacing of the skin.
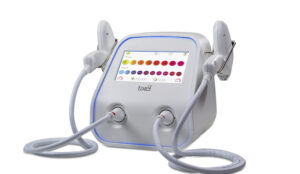
Aesthetic distribution company AZTEC Services has launched a new version of the Tixel device, called Tixel 2.