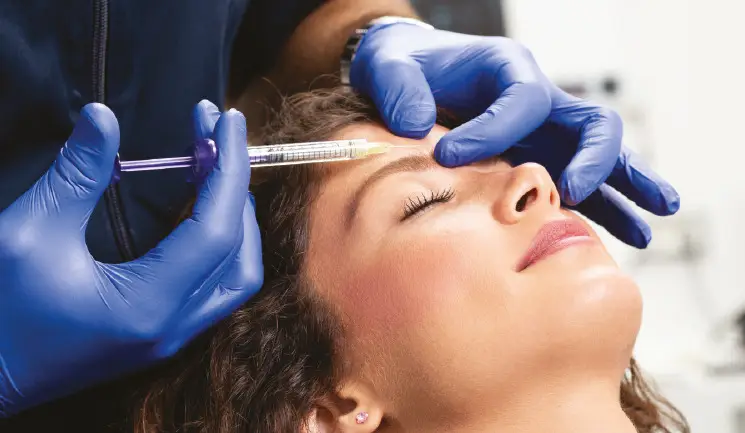
Nurse prescriber Lucy Williams discusses the potential causes of botulinum toxin resistance
To access this post, you must purchase Aesthetics Journal Membership – Annual Elite Membership, Aesthetics Journal Membership – Annual Enhanced Membership or Aesthetics Journal Membership – Basic Membership.
log in
log in