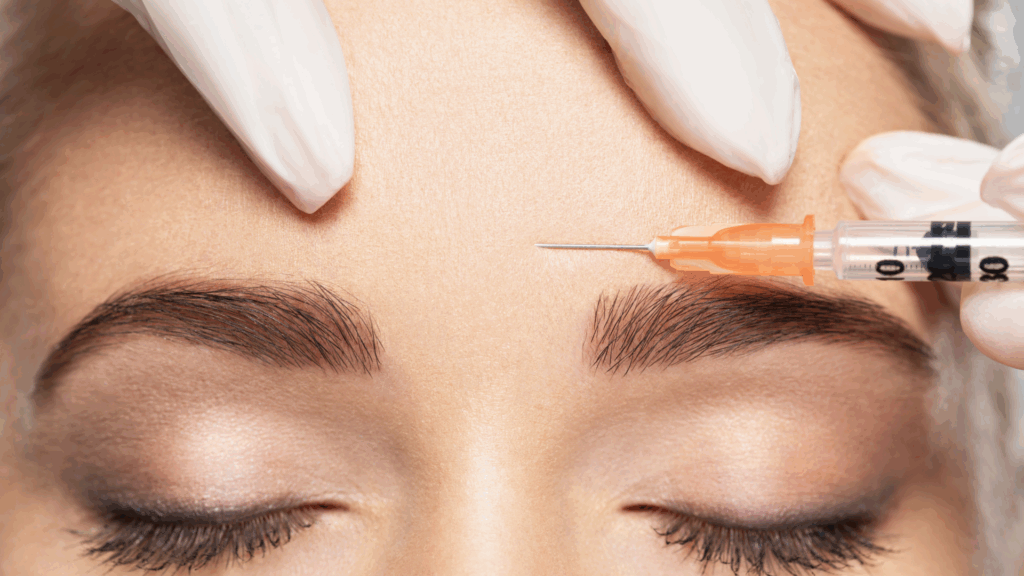
A BBC undercover investigation has found nurses and pharmacists putting patients at risk by supplying botulinum toxin without required face-to-face consultations.
In June, the Nursing and Midwifery Council (NMC) updated its position on the remote prescribing of prescription only medication (POMs). It confirmed that independent nurse and midwife prescribers are required to conduct a face-to-face consultation before prescribing POMs for non-surgical cosmetic procedures.
In Christchurch, Dorset, an undercover researcher posing as a beautician sought a prescription for botulinum toxin. Senior nurse prescriber Sally Jackson issued the prescription for £30, despite professional rules requiring an in-person consultation and a duty of care. The prescription details were exchanged via WhatsApp.
According to the BBC, Jackson also offered to prescribe additional vials under another patient’s name, enabling the undercover researcher to keep stock for patients without valid prescriptions – behaviour amounting to fraud. Jackson has ignored repeated requests for comment. When approached in person, she said only, “I am not interested.”
In East London, pharmacist prescriber Cornelius Agoye met a second undercover researcher, also posing as a beautician. He asked them to complete falsified consultation records to obtain prescriptions and stock. When contacted by the BBC, Agoye apologised, admitted falling below professional standards and said he had not intended to cause harm.
This year, 41 cases of botulism were reported between June and August, starting in County Durham and spreading to the North East, East of England and the Midlands. The outbreak was linked to unlicensed toxin and resulted in serious illness, prompting a crackdown by the Medicines and Healthcare products Regulatory Agency (MHRA) and warnings from the UK Health Security Agency (UKHSA).
Professor David Sines, chair of the Joint Council for Cosmetic Practitioners (JCCP), described the findings as “deeply concerning,” adding, “These checks are in place to prevent complications such as swelling, drooping, and other adverse reactions, and to ensure patients receive safe, appropriate care. When standards are bypassed, patients are left vulnerable and exposed to harm. Urgent action is needed to restore confidence and ensure the public can access safe, regulated care.”
The British Association of Medical Aesthetics (BAMAN) also condemned the practices, stating, “This behaviour is unethical, unsafe and in breach of the NMC’s standards. It compromises patient safety, undermines trust in the profession and damages the reputation of all nurses working with integrity in medical aesthetics.” BAMAN added that they are calling for urgent regulatory reform and remain committed to working with the NMC, MHRA and policymakers to ensure these failures are addressed systemically, not just individually.
The British College of Aesthetic Medicine (BCAM) issued a statement reiterating its commitment to patient safety. BCAM said its mission is to support clinicians in delivering high-quality medical aesthetics, to set and maintain the highest standards of practice and to protect patients by promoting safety and educating the public to make informed choices about cosmetic procedures.