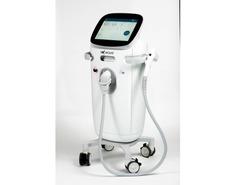
Medical device company Sofwave has received US Food and Drug Administration (FDA) clearance for a cellulite treatment to add to its SUPERB (synchronous ultrasound parallel beam) technology.
According to the company, SUPERB can already be used to treat wrinkles and fine lines on the face and neck, and this new clearance means cellulite treatment is now recommended to UK users, expanding the device’s potential.
Aesthetic practitioner and Sofwave user Dr Mahsa Saleki said, “We are thrilled that we can now treat cellulite with our Sofwave device. Non-invasive treatment of cellulite is one of the hardest concerns to treat, but also the most desired body treatments in medical aesthetics. Sofwave fills a gap in the market with incredible results.”