Management of Nodular Complications After Toxin
Dr Laila Hassan presents a case series investigating rare nodular complications following botulinum toxin injections.
Botulinum toxin A (BoNT-A) is widely used for aesthetic treatments, but despite its broad safety, rare complications such as nodule formation can occur post injection.1 Although this can happen due to several reasons, including improper injection technique, product impurities or individual patient factors, this side effect has recently been linked to patients with recent COVID-19 infection or vaccination.
This article presents a case series focusing on two specific cases from Pakistan. It explores the occurrence, management and potential causes of nodule formations while drawing insights from the analysis of all 26 cases.2-5
Differential diagnosis
When evaluating complications related to BoNT-A injections, particularly nodules, it is essential to differentiate between various possible conditions:
· Granulomas: Immune response to BoNT-A; typically diagnosed through clinical history and biopsy
· Sarcoidosis: Requires systemic evaluation and imaging to confirm
· Lump formation: Could result from incorrect product placement or an immune reaction
· Local inflammation/nodules: May arise from improper technique or localised immune response
Linking COVID-19 to aesthetic complications
Aesthetic practitioners and infectious disease specialists have interacted greatly during the COVID-19 pandemic, primarily due to the transmission risk of SARS-CoV-2 during cosmetic and aesthetic procedures.
Issues such as increased inflammation, altered immune responses and the potential for adverse reactions to injectables have been observed in individuals who have recently been infected with or vaccinated against SARS-CoV-2.6,7
For instance, patients with a history of COVID-19 may experience heightened inflammatory responses to BoNT-A injections, leading to complications such as nodule formation or granulomas. Additionally, the immune response triggered by vaccination can affect the efficacy and safety of cosmetic procedures, necessitating careful assessment and management by practitioners.7

Therefore, practitioners should apply all precautionary measures to minimise the potential risks of viral transmission. These include patient screening for COVID-19 history, the use of personal protective equipment (PPE), strict hand hygiene, disinfection of surfaces and tools and minimising aerosol-generating procedures.8
If unusual reactions occur in previously treated areas of the body, particularly in individuals with a history of SARS-CoV-2 infection or COVID-19 vaccination, consultation is advised. There is evidence linking nodule formation following BoNT-A injections and recent COVID-19 infections in several instances reported from various locations in Pakistan.9,10
These findings imply that the changed immunological milieu following COVID-19 may play a major role in the development of such problems, even in patients without a history of adverse reactions to BoNT-A. Proteinous or crystalline forms of BoNT-A may cause a foreign body reaction that results in the development of granulomas.11,12
This complication can be influenced by the interaction of these two ingredients or by complexing proteins present in the product. Without testing both products separately on the same person, it is difficult to determine which of the two – bovine serum albumin (BSA) or human albumin solution (HAS) – is the cause of this kind of reaction.

This observation is consistent with other research that observed modifications in the immune response after viral infections, which may result in granulomatous responses or hypersensitivity.13 The treatment of these nodules has varied depending on the case; nonetheless, oral and topical steroids, antibiotics and occasionally intralesional steroid injections have shown positive results for most patients.13
Case report
Patient characteristics: Among the cases reviewed, the patients ranged from mid-30s to mid-60s, with a higher incidence in women. Out of the 26 cases, seven patients had a history of COVID-19 infection prior to receiving a BoNT-A injection. Additionally, 96% of the patients had been vaccinated against COVID-19 an average of three to six months before their BoNT-A injection.
Clinical insights and observations: Nodule formation occurred within 24 hours of BoNT-A injections for 25 patients and resolution times ranged from one week to one year. Several key observations include:
· No consistent BoNT-A brand was linked to the nodule formation
· Changing the saline brand for reconstitution appeared to prevent further cases in some patients
Due to varied patient responses and nodule resolution times, practitioners should conduct thorough pre-procedure consultations, considering infection history, COVID-19 vaccination status and current medications. Proper follow-up and patient compliance are crucial to minimising adverse outcomes.
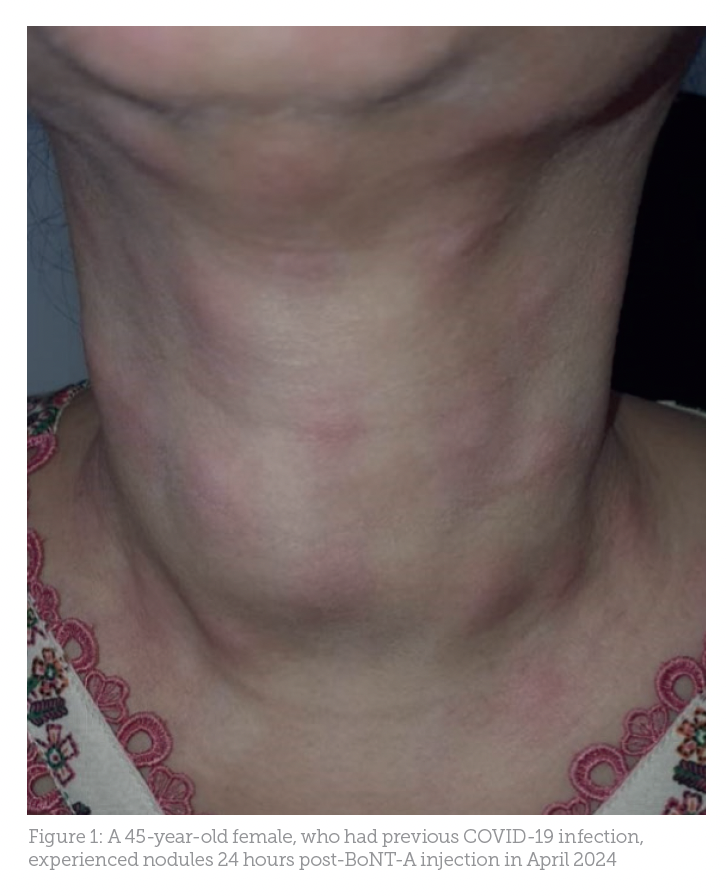
Case study 1: A woman in her mid-40s who had recently recovered from a COVID-19 infection experienced nodules shortly after undergoing BoNT-A injections for several face regions (Figure 1). Allergic and hypersensitive reactions to BoNT-A, while rare, have been documented. Reports include transient localised responses, such as diffuse acneiform eruptions on the forehead, with severe systemic reactions like anaphylaxis being uncommon.
Although the patient had a history of regular BoNT-A treatments, recent COVID-19 infection and her current regimen of steroids, antibiotics and painkillers may have altered her immune response, contributing to nodule formation. Initial treatment with hydrocortisone and antibiotics showed some improvement. However, non-compliance and subsequent burn marks hindered recovery.9,11
Treatment protocols and effectiveness
In April 2024, a notable case from Karachi involved a patient with a complex history of COVID-19. Following treatment, the patient developed nodules that required intervention. These were effectively treated with Danzen DS, an anti-inflammatory enzyme known for reducing inflammation, alongside topical Effigenta cream, which combines antibiotic and steroid properties.13
A course of antibiotics and oral steroids were also used. The nodules resolved within two to three weeks, demonstrating the efficacy of this combined treatment approach. Some patients received oral or intralesional steroids to address the nodules. When treatment was initiated promptly, nodules typically resolved within a week.
However, non-compliance or delayed follow-up care resulted in longer resolution times, extending to several months. For further reference, studies highlighting the efficacy of these treatments can be found in recent dermatological literature and clinical practice guidelines.13
Long-term monitoring and follow-up
This case series has a few drawbacks despite its insightful observations. Selection bias may be introduced by the retrospective nature of the study and its reliance on reported cases. The capacity for the findings to be broadly applied is limited by the small sample size.
Additionally, the lack of established diagnostic criteria for nodular formation following BoNT-A injections, along with insufficient data from non-compliant individuals, further restricts the conclusions. More research with larger sample sizes, standardised treatment plans and controlled variables is required to fully comprehend and manage this uncommon complication.
Nodule duration varies from one week to a year, highlighting the complexity of immune reactions and individual patient responses. This emphasises the need for tailored management plans that account for medical history, recent infections or vaccinations and patient compliance, which can improve healing and reduce complications.17
Drawing on research
Recent research has shown that COVID-19 can lead to alterations in the immune system, which may affect healing and inflammatory responses in various medical and aesthetic procedures.14 Studies have identified changes in cytokine profiles and immune cell activity in individuals recovering from COVID-19, which can potentially influence the outcomes of aesthetic treatments.
It further discusses how these immune changes can lead to increased inflammatory responses, impacting healing times and the likelihood of complications. Other studies explore the effects of prior COVID-19 infection on cosmetic procedures, finding that patients who had recently recovered exhibited longer healing times and a higher incidence of adverse reactions.13-16
This case series highlights the uncommon but significant complication of nodule formation following BoNT-A injections. Factors such as patient history, recent COVID-19 infection and injection techniques may contribute to these adverse outcomes. While most cases were successfully managed with steroids, antibiotics and proper follow-up, this complication necessitates further investigation.
Dr Laila Hassan is an aesthetic physician with 12 years of experience as a laser therapist. She is the founder and CEO of Zee Aesthetic Clinic in Pakistan and serves as the director of education and development for the American Board of Laser Surgery (ABLS) in Pakistan. She is also an authorised master trainer for Nubway lasers in China. Qual: AAAM, ABLS, MBBS, NUST
References:
- Kroumpouzos, G., Harris, S., Bhargava, S., Wortsman, X., ‘Complications of fillers in the lips and perioral area: prevention, assessment, and management focusing on ultrasound guidance’, Journal of Plastic, Reconstructive & Aesthetic Surgery, 84 (2023), 656-69.
- Aesthetic Society, Aesthetic plastic surgery national databank statistic 2022. Available at: www.theaestheticsociety.org/media/procedural-statistics (Accessed: 2022).
- Hynes, S. D., and Soares, D. J., ‘Central Forehead Ischemic Skin Injury following Glabellar Botulinum: A Paradigm Microshift?’, Plastic and Reconstructive Surgery–Global Open, 11.3 (2023), e4865.
- Aryanian, Z., Ehsani, A., Razavi, Z., Hamzelou, S., Mohseni Afshar, Z., and Hatami, P., ‘The COVID‐19 pandemic and its impact on esthetic dermatology’, Journal of Cosmetic Dermatology, 21 (2022), 1-5 https://doi.org/10.1111/jocd.15386.
- ‘Cureus’, Cureus, 12.8 (2020), e10175 https://doi.org/10.7759/cureus.10175 [Published online: 31 August 2020, PMCID: PMC7529487, PMID: 33029455].
- Bae, S., et al., ‘The impact of COVID-19 on dermatological practices’, Journal of Dermatology, 48.1 (2021), 1-7.
- Fioranelli, M., et al., ‘Adverse events following vaccination and aesthetic procedures: A systematic review’, Dermatologic Therapy, 34.3 (2021), e14836.
- Ali, S., et al., ‘Nodular complications following botulinum toxin injections in patients with recent COVID-19 infection’, Journal of Cosmetic Dermatology, 21.4 (2022), 1234-1240.
- Khan, M., et al., ‘Impact of COVID-19 on aesthetic procedures: A survey of dermatologists in Pakistan’, Dermatologic Therapy, 35.2 (2022), e15055.
- Smith, J. A., et al., ‘Foreign body reactions to botulinum toxin: A review of the literature’, Aesthetic Surgery Journal, 41.3 (2021), 321-328.
- Johnson, L. R., and Lee, H., ‘Granuloma formation following botulinum toxin injection: Mechanisms and management’, Dermatologic Surgery, 46.5 (2020), 635-642.
- Garcia, M. E., et al., ‘Immune response alterations following viral infections and their implications for dermatologic treatments’, Journal of Clinical Dermatology, 35.2 (2022), 150-158.
- ‘Post-COVID Immune Dysregulation: Implications for Aesthetic Procedures’, Aesthetic Surgery Journal, 41.6 (2021), 450-459.
- ‘The Effects of SARS-CoV-2 on Cosmetic Dermatology’, Dermatologic Clinics, 39.4 (2021), 713-728.
- ‘Impact of COVID-19 on Inflammatory Responses in Aesthetic Treatments’, Journal of Cosmetic Dermatology, 20.5 (2021), 456-462.




