The Advertising Standards Authority (ASA) has issued rulings on adverts for weight loss medication.
The ASA has upheld nine complaints against online advertisers promoting weight loss injections, classified as prescription only medicines (POMs), to the public, reinforcing that direct or indirect promotion is unlawful.
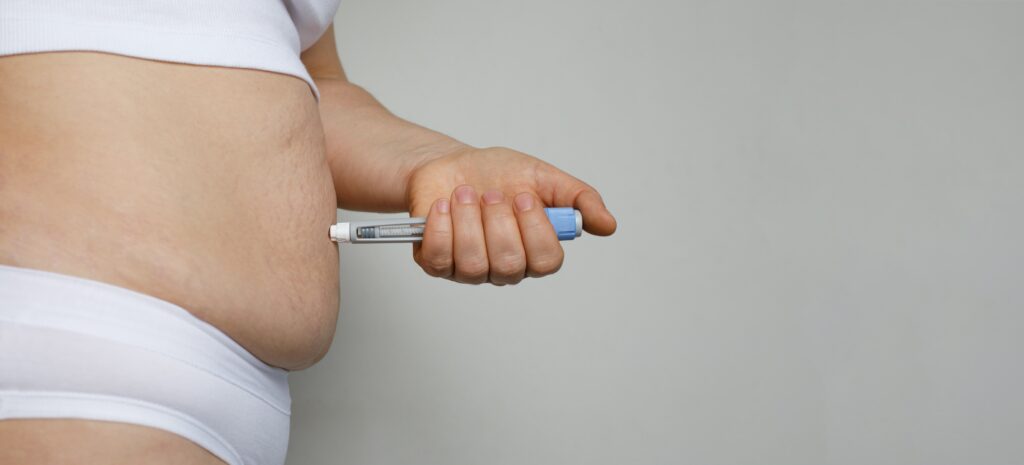
The ASA clarified that, in addition to prohibiting the naming of weight-loss drugs, the use of claims and imagery that indirectly promote such medications is also forbidden. The rulings target content that used phrases like “Obesity treatment jab,” “weight loss pen,” “GLP-1” and visuals such as injection pens or liquid vials, even when the drug names were omitted.
Jessica Tye, regulatory projects manager at the ASA, commented, “These nine rulings make crystal clear that all injectable forms of weight-loss medication are POMs and can’t be advertised to the public. We’ll be continuing to carry out extensive monitoring and enforcement work in this sector, and will take swift action against any breaches of the rules.”
Swedish healthcare brand Yazen was among the companies recently ruled against by the ASA. Fredrik Meurling, CEO and founder of Yazen, said, “We respect the ASA’s ruling and are open to taking guidance from the ASA in our future marketing efforts for the UK.”