
FDA warns against needle-free injector pens
The US Food and Drug Administration (FDA) has issued a safety communication regarding the use of needle-free injection devices for fillers.

The US Food and Drug Administration (FDA) has issued a safety communication regarding the use of needle-free injection devices for fillers.
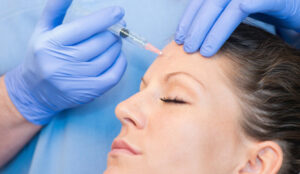
A study conducted by the Department of Dermatology at the Feinberg School of Medicine, Northwestern University, has suggested that cannulas are less likely to cause a vascular occlusion than needles when injecting filler.

Dr Ahmed El Houssieny reviews the evidence for aspiration when using dermal fillers and suggests ways to minimise risk of vascular compromise

On October 12, aesthetic manufacturer Merz Aesthetics invited eight aesthetic practitioners to attend the first Belotero Lips London Masterclass hosted by nurse prescriber and Merz Innovation Partner Lee Garrett.
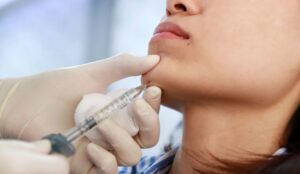
Aesthetic training provider Cosmetic Courses is now offering medical professionals training in the safe and effective use of Juvéderm Volux.

Global medical technology company Becton Dickinson (BD) has launched a glass, pre-fillable syringe for the administration of viscous solutions such as dermal fillers, called the BD Hylok.
We bring you the most up-to-date information for the Aesthetics community across a variety of media, including; news, webinars, podcasts, the Journal, reports, interviews and more!
aesthetics Journal
The Aesthetics Journal is the leading monthly publication for the medical aesthetics specialty. It is peer reviewed and spans an incredible volume of topics. Become a member today to receive the monthly print and digital Journal.
0203 096 1228
2nd Floor, Regal House, 70 London Road
Twickenham TW1 3QS, United Kingdom
© 2023 Easyfairs UK Ltd | Registered in England No. 05067979