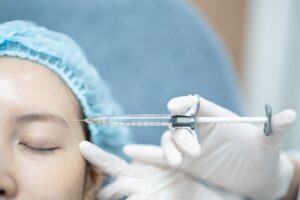
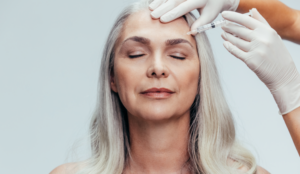
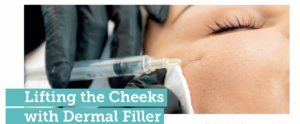
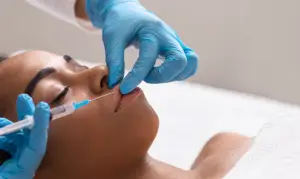
New research highlights ultrasound to prevent complications
New data has suggested ultrasound imaging as a means of minimising vascular risks during dermal filler procedures. Researchers used ultrasound to assess 100 cases of adverse filler outcomes across centres